 On 1st April ORPHANET DATA FOR RARE DISEASES 2 project was launched. Following a pilot phase in 2022, its goal is to contribute to meet the ambitions set by rare 2030 concerning data. Despite the fact that the Orphanet nomenclature already exists and is freely available in computable formats, the real-life implementation in health information systems is challenging due to the heterogeneity of coding systems and practices, and tools. The experience from the RD-CODE project , supporting diverse implementation models in four MS, taught us that local support in local language for coders and technical teams is necessary to achieve proper implementation in compliance with good practice guidelines for coding and to therefore increase data quality and comparability. To address those needs, it is important to maintain the Orphanet nomenclature of rare diseases, and to improve it building on the well organised and structured rare disease expertise laying in ERNs, to increase its interoperability with other terminologiesin use in different countries and in registries, and to contribute to the adoption and implementation,starting by the hospitals involved in ERNs by setting up a Network of National Orphanet Nomenclature Hubs in 19 MS and finally facilitate evidence-based decision through exploitation of the Orphanet Knowledge base linked to the ORPHAcodes. Read more here.
On 1st April ORPHANET DATA FOR RARE DISEASES 2 project was launched. Following a pilot phase in 2022, its goal is to contribute to meet the ambitions set by rare 2030 concerning data. Despite the fact that the Orphanet nomenclature already exists and is freely available in computable formats, the real-life implementation in health information systems is challenging due to the heterogeneity of coding systems and practices, and tools. The experience from the RD-CODE project , supporting diverse implementation models in four MS, taught us that local support in local language for coders and technical teams is necessary to achieve proper implementation in compliance with good practice guidelines for coding and to therefore increase data quality and comparability. To address those needs, it is important to maintain the Orphanet nomenclature of rare diseases, and to improve it building on the well organised and structured rare disease expertise laying in ERNs, to increase its interoperability with other terminologiesin use in different countries and in registries, and to contribute to the adoption and implementation,starting by the hospitals involved in ERNs by setting up a Network of National Orphanet Nomenclature Hubs in 19 MS and finally facilitate evidence-based decision through exploitation of the Orphanet Knowledge base linked to the ORPHAcodes. Read more here.
Publications
 Phelan-McDermid syndrome (PMS) is a rare genetic condition caused by a deletion encompassing the 22q13.3 region or a pathogenic variant of the gene SHANK3. The clinical presentation is variable, but main characteristics include global developmental delay/intellectual disability (ID), marked speech impairment or delay, along with other features like hypotonia and somatic or psychiatric comorbidities. This report delineates mental health, developmental and behavioural themes across the lifetime of individuals with PMS as informed by parents/caregivers, experts, and other key professionals involved in PMS care. In this report several recommendations based on the available literature concerning mental health and behaviour in PMS are put forward. Additionally, this article aims to improve the awareness of the importance of considering developmental level of the individual with PMS when assessing mental health and behavioural issues. Understanding how the discrepancy between developmental level and chronological age may impact concerning behaviours offers insight into the meaning of those behaviours and informs care for individuals with PMS, enabling clinicians to address unmet (mental health) care needs and improve quality of life. Read the full article here.
Phelan-McDermid syndrome (PMS) is a rare genetic condition caused by a deletion encompassing the 22q13.3 region or a pathogenic variant of the gene SHANK3. The clinical presentation is variable, but main characteristics include global developmental delay/intellectual disability (ID), marked speech impairment or delay, along with other features like hypotonia and somatic or psychiatric comorbidities. This report delineates mental health, developmental and behavioural themes across the lifetime of individuals with PMS as informed by parents/caregivers, experts, and other key professionals involved in PMS care. In this report several recommendations based on the available literature concerning mental health and behaviour in PMS are put forward. Additionally, this article aims to improve the awareness of the importance of considering developmental level of the individual with PMS when assessing mental health and behavioural issues. Understanding how the discrepancy between developmental level and chronological age may impact concerning behaviours offers insight into the meaning of those behaviours and informs care for individuals with PMS, enabling clinicians to address unmet (mental health) care needs and improve quality of life. Read the full article here.
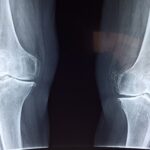 Multiple epiphyseal dysplasia, which affects the epiphysis of long bones, can show autosomal dominant and autosomal recessive inheritance patterns. The symptoms typically appear in childhood, although they sometimes do not show symptoms until adulthood. The goals of treatment in children are to prevent the early onset of osteoarthritis, improve function, and educate patients and their families about the natural history and genetic basis of the disease. Some patients present to the clinic with only non-healing and unidentified joint pain. Although multiple epiphyseal dysplasia type 5 is a rare disease with autosomal dominant inheritance in general, it can also be observed with de novo mutation, although very rarely, without a family history.
Multiple epiphyseal dysplasia, which affects the epiphysis of long bones, can show autosomal dominant and autosomal recessive inheritance patterns. The symptoms typically appear in childhood, although they sometimes do not show symptoms until adulthood. The goals of treatment in children are to prevent the early onset of osteoarthritis, improve function, and educate patients and their families about the natural history and genetic basis of the disease. Some patients present to the clinic with only non-healing and unidentified joint pain. Although multiple epiphyseal dysplasia type 5 is a rare disease with autosomal dominant inheritance in general, it can also be observed with de novo mutation, although very rarely, without a family history.
7-years-old male patient is admitted to a orthopedics outpatient clinic with complaints of joint pain, fatigue, and pain in the knees and ankles that had lasted for about 3 years. In the laboratory, there is no obvious finding other than vitamin D deficiency. The epiphyses, especially in the ankle, are dysplasic on Xray. After genetic tests multiple epiphyseal dysplasia type 5 is detected, with de novo mutation, without family histories.
Multiple epiphyseal dysplasia type 5 is a rare disease. It should be kept in mind that skeletal dysplasia should also be evaluated, although it is rarely seen in patients with persistent joint pain. Thus, it is possible to slow down the progression with early diagnosis of the patient and minimize the early surgical requirements. Read the full article here.
 Emerging machine learning (ML) technologies have the potential to significantly improve the research and treatment of rare diseases, which constitute a vast set of diseases that affect a small proportion of the total population. Artificial Intelligence (AI) algorithms can help to quickly identify patterns and associations that would be difficult or impossible for human analysts to detect. Predictive modeling techniques, such as deep learning, have been used to forecast the progression of rare diseases, enabling the development of more targeted treatments. Moreover, AI has also shown promise in the field of drug development for rare diseases with the identification of subpopulations of patients who may be most likely to respond to a particular drug. This report aims to highlight the achievements of AI algorithms in the study of rare diseases in the past decade and advise researchers on which methods have proven to be most effective. The review will focus on specific rare diseases, as defined by a prevalence rate that does not exceed 1-9/100,000 on Orphanet, and will examine which AI methods have been most successful in their study. Read the full article here.
Emerging machine learning (ML) technologies have the potential to significantly improve the research and treatment of rare diseases, which constitute a vast set of diseases that affect a small proportion of the total population. Artificial Intelligence (AI) algorithms can help to quickly identify patterns and associations that would be difficult or impossible for human analysts to detect. Predictive modeling techniques, such as deep learning, have been used to forecast the progression of rare diseases, enabling the development of more targeted treatments. Moreover, AI has also shown promise in the field of drug development for rare diseases with the identification of subpopulations of patients who may be most likely to respond to a particular drug. This report aims to highlight the achievements of AI algorithms in the study of rare diseases in the past decade and advise researchers on which methods have proven to be most effective. The review will focus on specific rare diseases, as defined by a prevalence rate that does not exceed 1-9/100,000 on Orphanet, and will examine which AI methods have been most successful in their study. Read the full article here.
Review and standard operating procedures for collection of biospecimens and analysis of biomarkers in new onset refractory status epilepticus
 New onset refractory status epilepticus (NORSE), including its subtype with a preceding febrile illness known as febrile infection-related epilepsy syndrome (FIRES), is one of the most severe forms of status epilepticus. The exact causes of NORSE are currently unknown, and there is so far no disease-specific therapy. Identifying the underlying pathophysiology and discovering specific biomarkers, whether immunologic, infectious, genetic, or other, may help physicians in the management of patients with NORSE. A broad spectrum of biomarkers has been proposed for status epilepticus patients, some of which were evaluated for patients with NORSE. Nonetheless, none has been validated, due to significant variabilities in study cohorts, collected biospecimens, applied analytical methods, and defined outcome endpoints, and to small sample sizes. The NORSE Institute established an open NORSE/FIRES biorepository for health-related data and biological samples allowing the collection of biospecimens worldwide, promoting multicenter research and sharing of data and specimens. In this report are standard operating procedures for biospecimen collection and biobanking in this rare condition suggested. Also are criteria for the appropriate use of previously collected biospecimens proposed. It is predicted that the widespread use of standardized procedures will reduce heterogeneity, facilitate the future identification of validated biomarkers for NORSE, and provide a better understanding of the pathophysiology and best clinical management for these patients. Read the full article here.
New onset refractory status epilepticus (NORSE), including its subtype with a preceding febrile illness known as febrile infection-related epilepsy syndrome (FIRES), is one of the most severe forms of status epilepticus. The exact causes of NORSE are currently unknown, and there is so far no disease-specific therapy. Identifying the underlying pathophysiology and discovering specific biomarkers, whether immunologic, infectious, genetic, or other, may help physicians in the management of patients with NORSE. A broad spectrum of biomarkers has been proposed for status epilepticus patients, some of which were evaluated for patients with NORSE. Nonetheless, none has been validated, due to significant variabilities in study cohorts, collected biospecimens, applied analytical methods, and defined outcome endpoints, and to small sample sizes. The NORSE Institute established an open NORSE/FIRES biorepository for health-related data and biological samples allowing the collection of biospecimens worldwide, promoting multicenter research and sharing of data and specimens. In this report are standard operating procedures for biospecimen collection and biobanking in this rare condition suggested. Also are criteria for the appropriate use of previously collected biospecimens proposed. It is predicted that the widespread use of standardized procedures will reduce heterogeneity, facilitate the future identification of validated biomarkers for NORSE, and provide a better understanding of the pathophysiology and best clinical management for these patients. Read the full article here.
 Traditional clinical trials require tests and procedures that are administered in centralized clinical research sites, which are beyond the standard of care that patients receive for their rare and chronic diseases. The limited number of rare disease patients scattered around the world makes it particularly challenging to recruit participants and conduct these traditional clinical trials.
Traditional clinical trials require tests and procedures that are administered in centralized clinical research sites, which are beyond the standard of care that patients receive for their rare and chronic diseases. The limited number of rare disease patients scattered around the world makes it particularly challenging to recruit participants and conduct these traditional clinical trials.
Participating in clinical research can be burdensome, especially for children, the elderly, physically and cognitively impaired individuals who require transportation and caregiver assistance, or patients who live in remote locations or cannot afford transportation. In recent years, there is an increasing need to consider Decentralized Clinical Trials as a participant-centric approach that uses new technologies and innovative procedures for interaction with participants in the comfort of their home.
This report discusses the planning and conduct of Decentralized Clinical Trials, which can increase the quality of trials with a specific focus on rare diseases. Read the full article here.
Early postnatal administration of an AAV9 gene therapy is safe and efficacious in CLN3 disease
 CLN3 disease, caused by biallelic mutations in the CLN3 gene, is a rare pediatric neurodegenerative disease that has no cure or disease modifying treatment. The development of effective treatments has been hindered by a lack of etiological knowledge, but gene replacement has emerged as a promising therapeutic platform for such disorders. In this research a mouse model of CLN3 disease to test the safety and efficacy of a cerebrospinal fluid-delivered AAV9 gene therapy with a study design optimized for translatability is used. In this model, postnatal day one administration of the gene therapy virus resulted in robust expression of human CLN3 throughout the CNS over the 24-month duration of the study. A range of histopathological and behavioral parameters are assayed, with the therapy consistently and persistently rescuing a number of hallmarks of disease while being safe and well-tolerated. Together, the results show great promise for translation of the therapy into the clinic, prompting the launch of a first-in-human clinical trial. Read the full article here.
CLN3 disease, caused by biallelic mutations in the CLN3 gene, is a rare pediatric neurodegenerative disease that has no cure or disease modifying treatment. The development of effective treatments has been hindered by a lack of etiological knowledge, but gene replacement has emerged as a promising therapeutic platform for such disorders. In this research a mouse model of CLN3 disease to test the safety and efficacy of a cerebrospinal fluid-delivered AAV9 gene therapy with a study design optimized for translatability is used. In this model, postnatal day one administration of the gene therapy virus resulted in robust expression of human CLN3 throughout the CNS over the 24-month duration of the study. A range of histopathological and behavioral parameters are assayed, with the therapy consistently and persistently rescuing a number of hallmarks of disease while being safe and well-tolerated. Together, the results show great promise for translation of the therapy into the clinic, prompting the launch of a first-in-human clinical trial. Read the full article here.
 Acquired haemophilia A is a rare disease with an annual incidence of 1.48 per million. Based on clinical observations, in this article a higher incidence in southern Switzerland is suspected, and its aim is to provide local epidemiological data, and clinical information regarding diagnosis, treatment and outcome in this region. All adult patients with acquired haemophilia A treated between 2013 and 2019 in a medical establishment in southern Switzerland are included in the present retrospective analysis.
Acquired haemophilia A is a rare disease with an annual incidence of 1.48 per million. Based on clinical observations, in this article a higher incidence in southern Switzerland is suspected, and its aim is to provide local epidemiological data, and clinical information regarding diagnosis, treatment and outcome in this region. All adult patients with acquired haemophilia A treated between 2013 and 2019 in a medical establishment in southern Switzerland are included in the present retrospective analysis.
11 patients with acquired haemophilia A between 2013 and 2019 are treated, resulting in an annual incidence of 4.5 per million. Median delay from first symptoms to diagnosis was 4.5 days, and the median age at diagnosis is 79 years. Possible causative conditions were: pregnancy, polyarteritis nodosa, myelodysplastic syndrome, chronic human immunodeficiency virus (HIV), and HIV postexposure prophylaxis. In five patients no underlying or associated condition was identified. All patients had bleeding symptoms, 5/10 patients had major bleedings, and 7/10 patients were treated with bypassing agents. All patients received corticosteroids; 7/10 patients received immunosuppressive combination therapy.
Acquired haemophilia A is a rare disease, but manageable despite the advanced patient age and comorbidities. Its incidence in Southern Switzerland is higher than previously suspected. Read the full article here.
 For the 14th year in a row, the National Conference on Rare Diseases and Orphan Drugs, organized by the Institute for Rare Diseases, is about to be held. The event is an annual forum that brings together all stakeholders – medical professionals, patients, medical students, industry representatives and health authorities – to present and discuss the latest developments in the diagnosis, treatment and follow-up of rare diseases, the development of European reference networks and access to innovation.
For the 14th year in a row, the National Conference on Rare Diseases and Orphan Drugs, organized by the Institute for Rare Diseases, is about to be held. The event is an annual forum that brings together all stakeholders – medical professionals, patients, medical students, industry representatives and health authorities – to present and discuss the latest developments in the diagnosis, treatment and follow-up of rare diseases, the development of European reference networks and access to innovation.
Place: Hotel “Imperial” – Plovdiv
Early bird registration deadline: August 1, 2023
Late registration deadline: September 15, 2023
Deadline for submission of abstracts: September 1, 2023
To contact the organizing committee: congress@raredis.org
Additional information can be found at XIV NATIONAL CONFERENCE ON RARE DISEASES AND ORPHAN DRUGS – Virtual Congress Center (raredis.org)
Enhancing Equitable Access to Rare Disease Diagnosis and Treatment around the World: A Review of Evidence, Policies, and Challenges
 This document provides a comprehensive summary of evidence on the current situation of rare diseases (RDs) globally and regionally, including conditions, practices, policies, and regulations, as well as the challenges and barriers faced by RD patients, their families, and caregivers. The document builds on a review of academic literature and policies and a process of validation and feedback by a group of seven experts from across the globe. The document is divided into five main sections: methodology and objective; background and context; overview of the current situation and key challenges related to RDs covering six dimensions: burden of disease, patient journey, social impact, disease management, RD-related policies, and research and development; recommendations; and conclusions. The recommendations are derived from the discussion undertaken by the experts on the findings of this review and provide a set of actionable solutions to the challenges and barriers to improving access to RD diagnosis and treatment around the world. The recommendations can support critical decision-making, guiding efforts by a broad range of RDs stakeholders, including governments, international organizations, manufacturers, researchers, and patient advocacy groups. Read the full article here.
This document provides a comprehensive summary of evidence on the current situation of rare diseases (RDs) globally and regionally, including conditions, practices, policies, and regulations, as well as the challenges and barriers faced by RD patients, their families, and caregivers. The document builds on a review of academic literature and policies and a process of validation and feedback by a group of seven experts from across the globe. The document is divided into five main sections: methodology and objective; background and context; overview of the current situation and key challenges related to RDs covering six dimensions: burden of disease, patient journey, social impact, disease management, RD-related policies, and research and development; recommendations; and conclusions. The recommendations are derived from the discussion undertaken by the experts on the findings of this review and provide a set of actionable solutions to the challenges and barriers to improving access to RD diagnosis and treatment around the world. The recommendations can support critical decision-making, guiding efforts by a broad range of RDs stakeholders, including governments, international organizations, manufacturers, researchers, and patient advocacy groups. Read the full article here.
